Mark Paddock, Ph.D University of California, San Diego
I am currently focused on two main areas of research. The first focus is on the primary events in photosynthesis that involves chemistry shared between photosynthetic and respiratory enzymes. The second focus is on the novel CDGSH domain containing proteins and their mitochondrial associated function that has been shown to be related to diabetic therapies and human disease and longevity.

Photosynthesis is the process by which plants and some bacteria convert light energy into chemical energy. It is responsible for feeding almost all of life and is the major harvester of light energy. My interest in the area of photosynthesis is founded in its importance for performing fundamental chemistry involved in energy conversion and storage. In particular, my research has historically focused on the pathway for proton transfer into a catalytic site of an enzyme and the coupling of proton and electron transfer reactions in the photosynthetic reaction center from Rhodobacter sphaeroides. In addition, in collaboration with Prof. Okamura (Physics) we have investigated (i) the structural features of the bacterial reaction center that control the high quantum energy conversion and its directionality along the so called A-branch and (ii) its interaction between the mobile electron carrier, cytochrome c2, to the membrane bound RC. These investigations hold the promise to gain understanding of high efficiency solar energy conversion and protein-protein recognition required for controlling the ever dynamic chemical energy conversion that occurs in life.
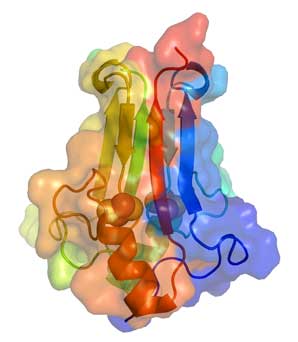
CDGSH Proteins and Mitochondria:
Mitochondria are known as the powerhouses of cells that perform respiration, the process in which nutrients are converted to usable energy in the cell. My interest in the area of mitochondrial function stems from its importance for quality of human life. In particular, my research has been on the physical and biochemical investigations of a new class of iron-sulfur proteins that contain a CDGSH iron sulfur binding domain. Early studies showed that a human CDGSH protein was a mitochondrial target of the thiazolidinedione type II diabetic treatments and hence was given the name mitoNEET where mito refers to its mitochondrial association and NEET was part of the protein sequence. A second human family member was shown to be associated with a neurological disease called Wolfram Syndrome 2 and its knockout in mice lead to a decreased lifespan and other neurological and skeletal problems resulting in a lower quality of life. Having established that the protein structures are novel, containing uniquely coordinated 2Fe-2S centers, in collaboration with Prof. Jennings (Department of Chemistry and Biochemistry) we plan to further investigate the functions, link to diabetes and gain further fundamental understanding of the unique properties of these proteins, which include EPR investigations of the coordination of the 2Fe-2S redox centers.
Department of Physics 0319
9500 Gilman Drive
University of California, San Diego
La Jolla, CA 92093-0319
email:mpaddock@ucsd.edu
phone: (858) 534-2504