Mark Paddock, Ph.D University of California, San Diego
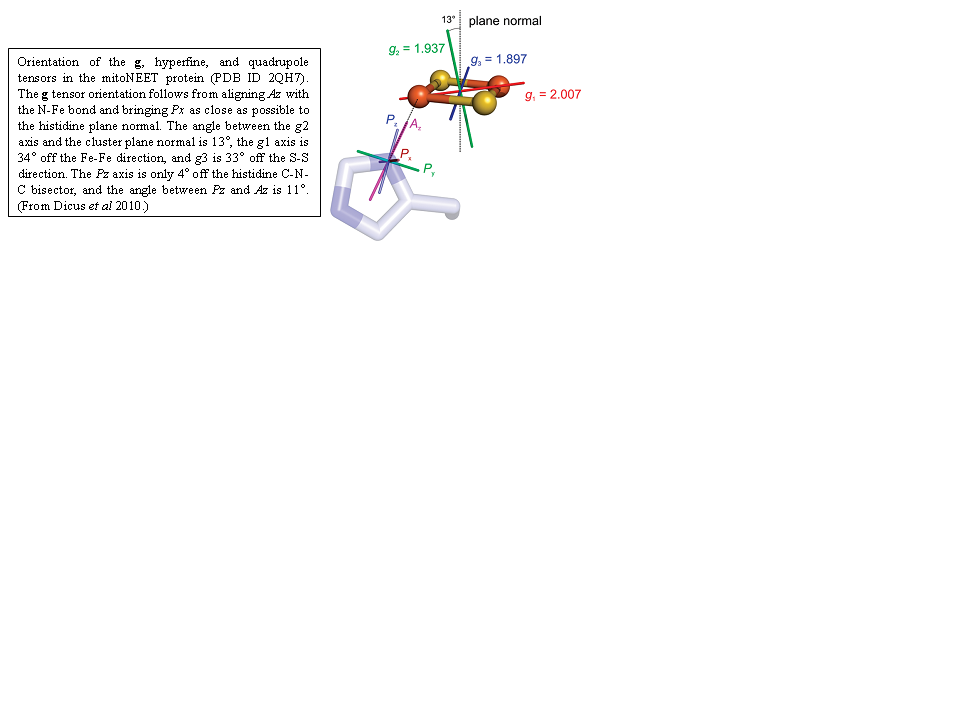
Human mitoNEET is a homodimeric iron-sulfur protein located in the outer mitochondrial membrane with each monomer harboring a [2Fe-2S] cluster with an unusual 3Cys-1His ligation. The His ligand is important for enabling cluster release and for tuning the redox potential. To determine details on the interaction between the single His ligand and the [2Fe-2S] center, multifrequency (X-, Ka-, and Q-band) and multitechnique (continuous-wave, electron spin-echo envelope modulation (ESEEM), pulsed electron-nuclear double resonance (ENDOR), and hyperfine sublevel correlation (HYSCORE)) electron paramagnetic resonance spectroscopy were used to investigate the cluster in its paramagnetic reduced [Fe(2+)Fe(3+)] (S = 1/2) state. It has a rhombic g tensor (2.007, 1.937, 1.897) with an average g value of 1.947 that falls between those of Rieske-type and ferredoxin-type [2Fe-2S] clusters. From simulation and least-squares fitting of the date, the absolute g tensor orientation with respect to the cluster was deduced (see Figure below). In X-band ENDOR and ESEEM spectra, a weakly coupled nitrogen is visible, most likely the N(epsilon) of the histidine in the protonated state. We find that the cluster is in a valence-localized state, where Fe(2+) is His-bound. The field-sweep spectra show evidence of intercluster dipolar coupling that can be simulated using an uncoupled spin model for each cluster (S(Fe(2+)) = 2, S(Fe(3+)) = 5/2). Thus, mitoNEET is dimeric in solution. These data and analyses serve as a paradigm for other His coordinated [2Fe-2S] centers such as those in Rieske centers involved in respiration.